BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

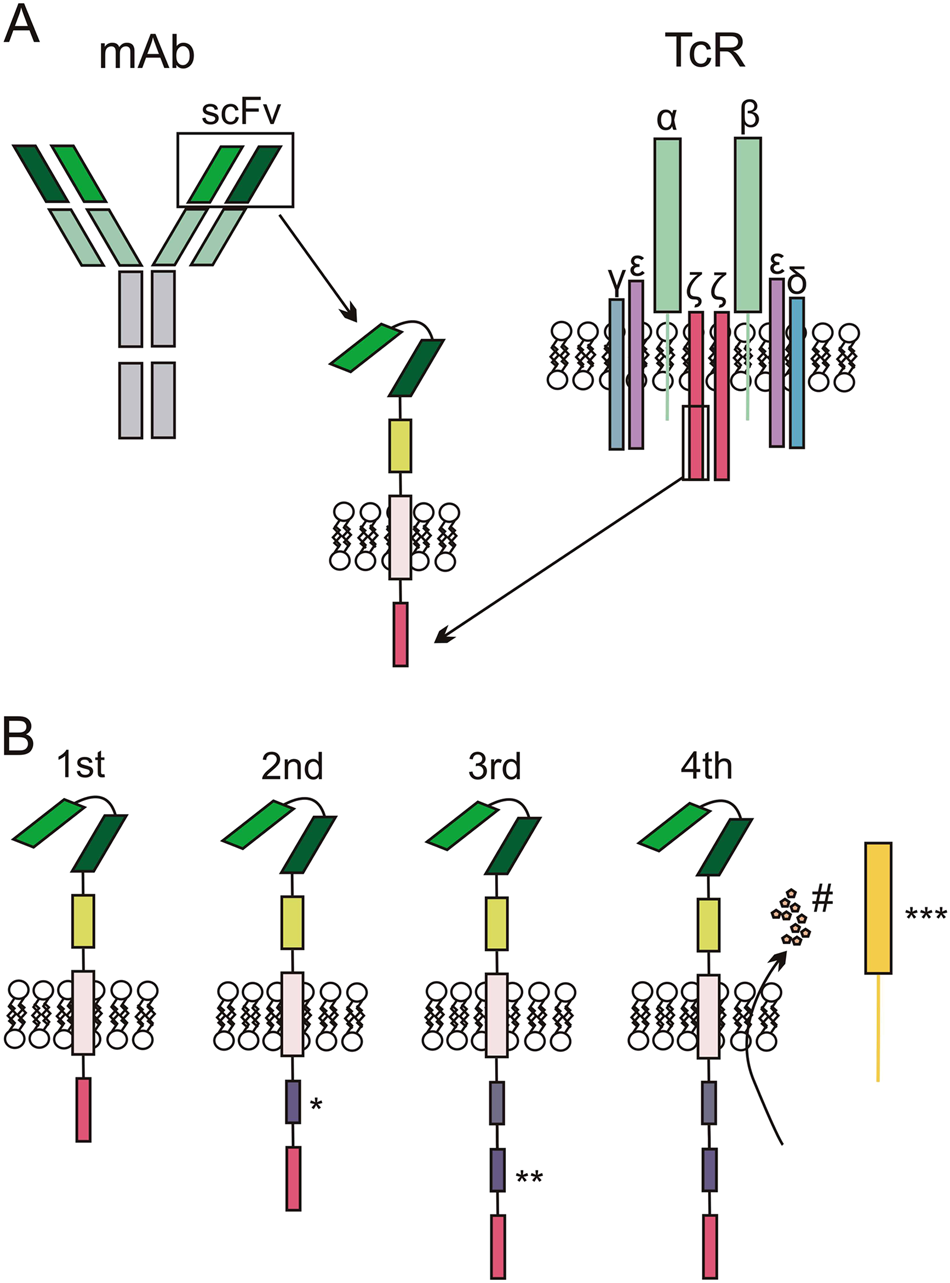
Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
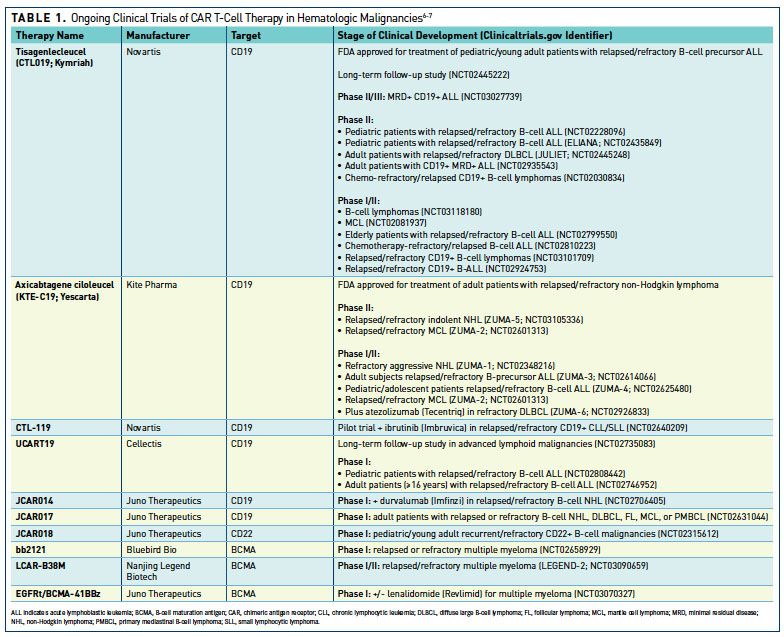
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

FDA Advisory Committee Recommends Approval of CAR-T Cell Therapy and Two New Biosimilars | Biosimilars Law Bulletin
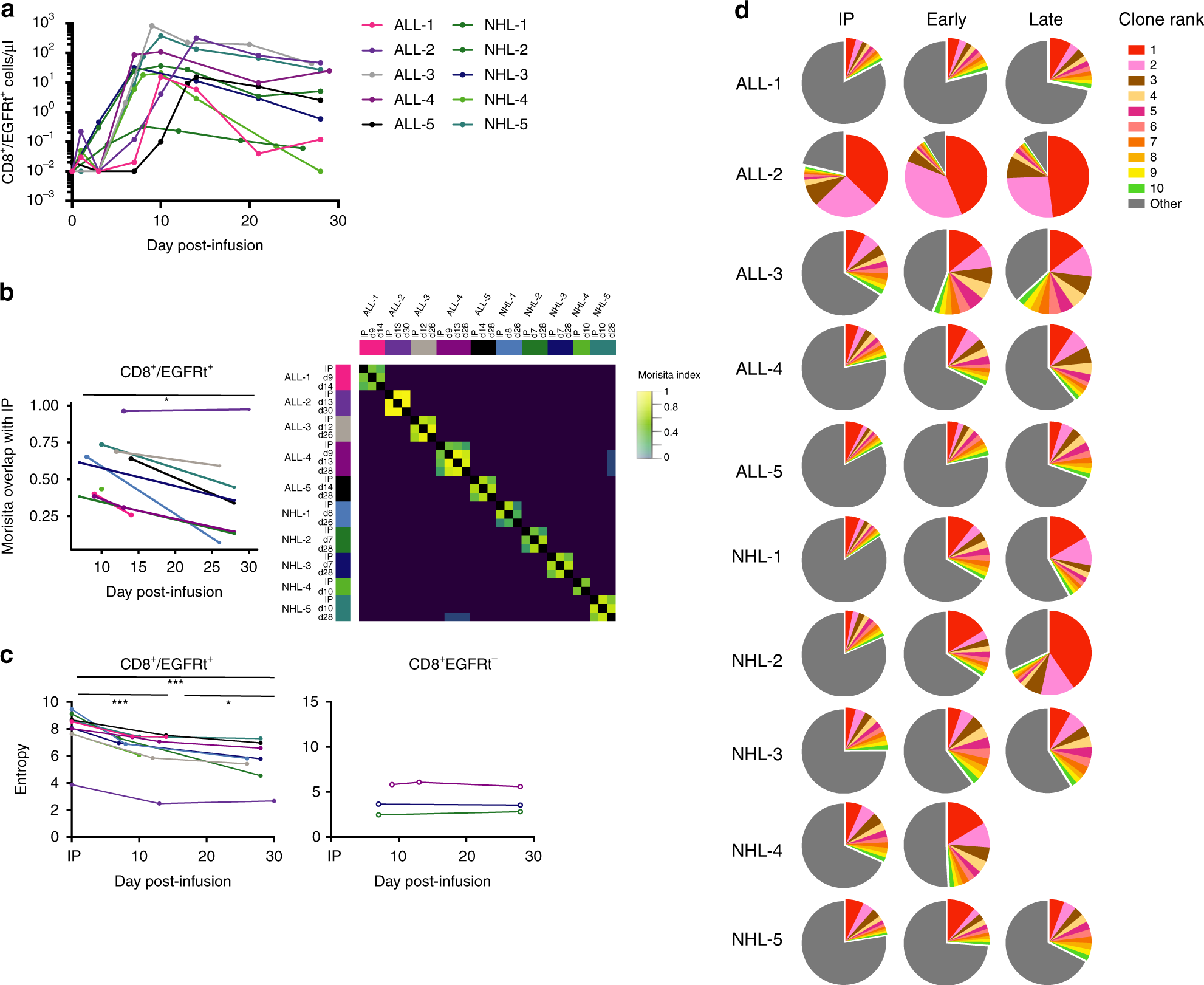
Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy | Nature Communications
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar